Triethyl phosphonoacetate
CAS 867-13-0, Cat. No EN300-52552
Reagent for Horner–Wadsworth–Emmons reaction
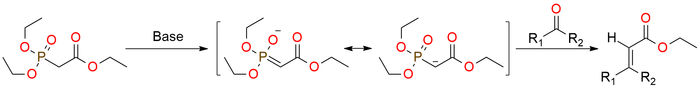
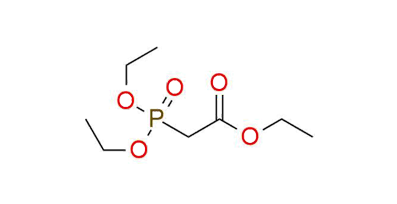
Triethyl phosphonoacetate is a reagent for the Horner–Wadsworth–Emmons (HWE) reaction1. It is a shelf-stable liquid under proper storage conditions. Deprotonation by diverse bases yields a carbanion, capable of reacting with carbonyl compounds, resulting in alkene formation. Notably, the reaction showcases a preference for the E-isomer, although this selectivity tends to be less pronounced with ketones to aldehydes. In the case of "sensitive" substrates, the HWE reaction can be carried out under Masamune-Roush conditions - utilizing LiCl and DBU in acetonitrile. This leads to the successful transformation of initial compounds into desired products with good yields.
Synonyms: acetic acid, (diethoxyphosphinyl)-, ethyl ester (9CI); acetic acid, phosphono-, triethyl ester (6CI, 7CI, 8CI); (diethoxyphosphinyl)acetic acid ethyl ester; (diethoxyphosphoryl)acetic acid ethyl ester; (ethoxycarbonylmethyl)diethoxyphosphine oxide; 2-(diethoxyphosphinyl)acetic acid ethyl ester; 2-phosphonoacetic acid triethyl ester; carbethoxymethyldiethyl phosphonate; diethyl 2-ethoxy-2-oxoethylphosphonate; diethyl carbethoxymethylphosphonate; diethyl ethoxycarbonylmethanephosphonate; diethyl phosphonoacetic acid ethyl ester; ethyl (diethoxyphosphinyl)acetate
Selected publication
1. Triethyl Phosphonoacetate.Abell A.; Bessières B. Encyclopedia of Reagents for Organic Synthesis 2006. DOI: 10.1002/047084289X.rt225.pub2
