PyAOP
CAS 156311-83-0, Cat. No EN300-7492597
Reagent for peptide synthesis, C/N/O nucleophiles triphosphorylation
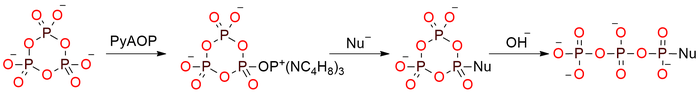
PyAOP was originally designed as an activator for carboxylic acids but recently found uses in the field of functionalization of intact trimetaphosphate1. It is a colorless crystals, soluble in CH2Cl2, CHCl3, DMF, DMSO, NMP, THF, CH3CN, and acetone2. As a reagent-activator for carboxylic acids in peptide synthesis, it doesn’t react with primary amines, in contrast to the corresponding uronium/aminium salts which lead to guanidino derivatives. PyAOP is among the most reactive coupling reagents with strong stability, which makes it less effective in automated solid-phase peptide synthesis. The functionalization of trimetaphosphate can be done with primary alcohols, amines, secondary amines, and Wittig reagents to form phosphorus ylide in moderate yield. The reagent can be applied to the triphosphorylation of nucleosides.
Synonyms: (7-azabenzotriazol-1-yloxy)tris (pyrrolidino)phosphonium hexafluorophosphate; tri-pyrrolidinyl[3H-1,2,3-triazolo (4,5-b)pyridin-3-yloxy]phosphonium hexafluorophosphate; tripyrrolidin-1-yl(triazolo[4,5-b]pyridin-3-yloxy)phosphonium hexafluorophosphate; PyAOP
Selected publication
1. Functionalization of Intact Trimetaphosphate: A Triphosphorylating Reagent for C, N, and O Nucleophiles.Shepard S.; Cummins C. J Am Chem Soc 2019, 141 (5), 1852–1856. DOI: 10.1021/jacs.8b12204
2. (7-Azabenzotriazol-1-Yloxy)Tris(Pyrrolidino)Phosphonium Hexafluorophosphate.Coste J.; Jouin P. Encyclopedia of Reagents for Organic Synthesis 2003. DOI: 10.1002/047084289X.rn00199
