Dimethyl P-(1-diazo-2-oxopropyl)phosphonate
CAS 90965-06-3, Cat. No EN300-133907
Reagent for Seyferth–Gilbert homologation
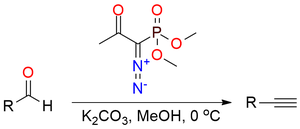
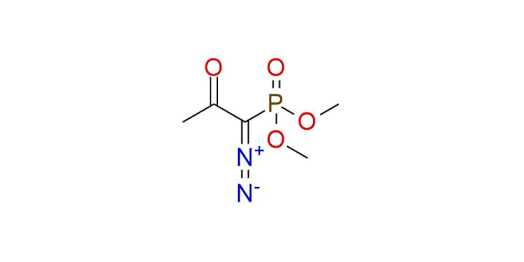
Dimethyl P-(1-diazo-2-oxopropyl)phosphonate (Ohira-Bestmann reagent) is primarily used in Seyferth–Gilbert homologation in terminal acetylenes synthesis1,2. It is a pale-yellow liquid soluble in most organic solvents but can slowly react with alcohols. The reagent forms vinylidene carbenes in the presence of a base and carbonyl compounds. In the case of aldehydes rearranging proceeds to alkynes and for ketones - to enol ethers. The choice between two variants of the Ohira-Bestmann reagent (methyl or ethyl ester) depends on what alcohol is used as media for the reaction. Besides homologation, the reagent can be used for 1,3-dipolar cycloaddition to form phosphoryl-substituted nitrogen heterocycles: pyrazoles, triazolines, oxazoles, thiazoles, etc.
Synonyms: dimethyl P-(1-diazo-2-oxopropyl)phosphonate (ACI); phosphonic acid, (1-diazo-2-oxopropyl)-, dimethyl ester (9CI); (1-diazo-2-oxopropyl)phosphonic acid dimethyl ester; 1-diazo-1-(dimethyl phosphono)acetone; 1-diazo-1-dimethoxyphosphoryl-propan-2-one; Bestmann reagent; Bestmann-Ohira reagent; dimethyl (1-azo-2-oxopropyl)phosphonate; dimethyl (1-azoacetonyl)phosphonate; dimethyl (1-diazo-2-oxopropyl)phosphonate; dimethyl (acetyldiazomethyl)phosphonate; Ohira's reagent; Ohira-Bestmann phosphonate; Ohira-Bestmann reagent; α-azido-α-(dimethyl phosphono)acetone
Selected publication
1. (1-Diazo-2-Oxopropyl)-Phosphonic Acid Dimethyl Ester.Gandon V.; Aubert C. Encyclopedia of Reagents for Organic Synthesis 2008. DOI: 10.1002/047084289X.rn00780
2. An Improved One-Pot Procedure for the Synthesis of Alkynes from Aldehydes.Müller S.; Liepold B.; Roth G.; Bestmann H. Synlett 1996, 1996 (06), 521–522. DOI: 10.1055/s-1996-5474
