Dimethyl 1,2,4,5-tetrazine-3,6-dicarboxylate
CAS 2166-14-5, Cat. No EN300-211397
Versatile building block in Retro-Diels-Alder reactions
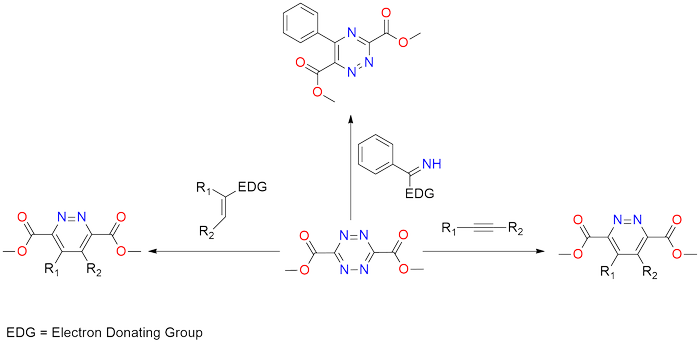
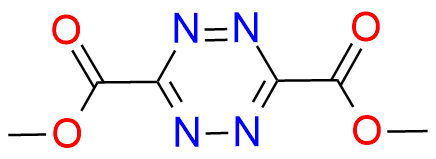
Dimethyl 1,2,4,5-tetrazine-3,6-dicarboxylate is a highly versatile heteroaromatic azadiene that has garnered considerable attention for its exceptional reactivity and its ability to engage in Retro-Diels-Alder reaction with a wide range of dienophiles and heterodienophiles[1]. This compound enables the synthesis of substituted 1,2-diazines, 1,2,4-triazines, pyrroles, pyridines, indolines, and related condensed heterocycles through cycloaddition reactions that have an operationally simple procedure[2], [3]. The Mildness of the reaction conditions and predictable positional selectivity, high level of functional group compatibility make this heteroaromatic azadiene perfect as a reagent for pharmaceutically important targets[1], [2], [4].
Synonyms: 3,6-dimethyl 1,2,4,5-tetrazine-3,6-dicarboxylate
Selected publication
1. The Retro–Diels–Alder Reaction Part II. Dienophiles with One or More Heteroatom.
Rickborn B. Organic Reactions 1998, 223–629. DOI: 10.1002/0471264180.or053.02
2. Dimethyl 1,2,4,5-Tetrazine-3,6-Dicarboxylate.
Boger D. L.; Zhang M.; Haider N. Encyclopedia of Reagents for Organic Synthesis 2006. DOI: 10.1002/047084289X.rd389.pub2
3. Preparation and diels-alder reaction of a reactive, electron-deficient heterocyclic azadiene: dimethyl 1,2,4,5-tetrazine-3,6-dicarboxylate. 1,2-diazine (dimethyl 4-phenyl-1,2-diazine-3,6-dicarboxylate) and pyrrole (dimethyl 3-phenylpyrrole-2,5-dicarboxylate) introduction.
Organic Syntheses 1992, 70, 79. DOI: 10.15227/orgsyn.070.0079
4. A Detailed, Convenient Preparation of Dimethyl 1,2,4,5-Tetrazine-3,6-Dicarboxylate.
N. Alonso et al. J Org Chem 1985, 50 (25), 5377–5379. DOI: 10.1021/jo00225a076
