Difluoro(trimethylsilyl)acetonitrile
CAS 1384170-26-6, Cat. No EN300-217202
Cyanodifluoromethylating reagent
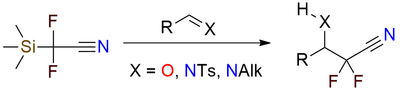
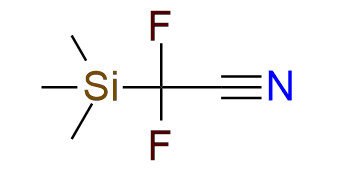
This reagent offers a convenient way to obtain organofluorine compounds for pharmaceutical and agrochemical chemistry[1], [2]. It is capable of nucleophilic cyanodifluoromethylating of aldehydes, N-tosylimines, N-alkylimines, and enamines under basic or acidic conditions[2]. The reagent is a bench-stable colorless liquid that is easy to work with. The nitrile group in the obtained compounds can be easily transformed into an amino or amide group, depending on the substrate (alcohol or protected amino group).
Synonyms: 2,2-Difluoro-2-(trimethylsilyl)acetonitrile
Selected publication
1. Silicon-Based Reagents for Difluoromethylation and Difluoromethylenation Reactions.
Krishnamoorthy S.; Prakash G. Synthesis 2017, 49 (15), 3394–3406. DOI: 10.1055/s-0036-1588489
2. Difluoro(Trimethylsilyl)Acetonitrile: Synthesis and Fluoroalkylation Reactions.
Kosobokov M. D.; Dilman A. D.; Levin V. V.; Struchkova M. I. J Org Chem 2012, 77 (13), 5850–5855. DOI: 10.1021/jo301094b
