DAST
CAS 38078-09-0, Cat. No EN300-97056
Reagent for deoxofluorination
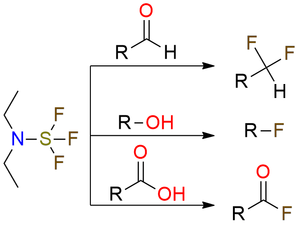
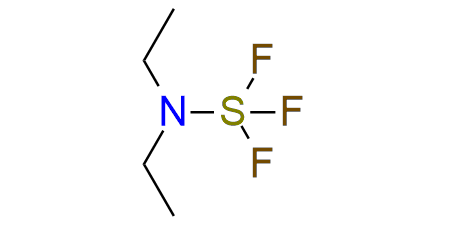
DAST is a reagent for deoxofluorination that is still actual in organic synthesis. It is a yellow liquid that is relatively safe compared to SF4 but requires careful working. DAST can convert alcohols to fluorides aldehydes and unhindered ketones to difluorides and finally carboxylic acids to acyl fluoride, and sulfoxides to α-fluoro sulfides with high yields. In some cases, DAST can promote cationic rearrangements (Wagner-Meerwein rearrangement, pinacol rearrangement) which is important to remember.
Synonyms: diethylaminosulfur trifluoride, Sulfur, (N-ethylethanaminato)trifluoro-, (T-4)-, Ethanamine, N-ethyl-, sulfur complex, (T-4), (N-Ethylethanaminato)trifluorosulfur, trifluoro(diethylamino)sulfur, (Diethylamino)sulfur trifluoride
Selected publication
1. Diethylaminosulfurtrifluoride.
Fauq A. H.; Singh R. P.; Meshri D. T. Encyclopedia of Reagents for Organic Synthesis 2006. DOI: 10.1002/047084289X.rd175
2. Achievements in Fluorination Using Variable Reagents through a Deoxyfluorination Reaction.
Aggarwal T.; Sushmita; Verma A. K. Organic Chemistry Frontiers 2021, 8 (22), 6452–6468. DOI: 10.1039/D1QO00952D
3. Enantiospecific Deoxyfluorination of Cyclic α-OH-β-Ketoesters.
Mairhofer C.; Haider V.; Bögl T.; Waser M. Org Biomol Chem 2021, 19 (1), 162–165. DOI: 10.1039/D0OB02152K
