Bredereck’s reagent
CAS 5815-08-7, Cat. No EN300-80797
Reagent for aminomethylenating
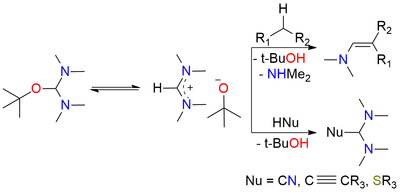
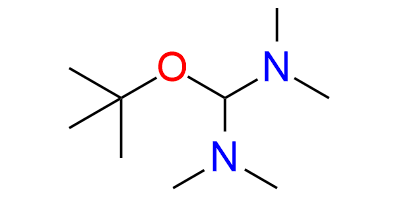
Bredereck's reagent is an aminal ester recognized for its reactivity as a powerful aminomethylenating reagent, often employed for the formylation of CH2- and NH2-acidic compounds. It readily reacts with CH2-acidic ketones, including straightforward dialkyl ketones, aryl alkyl ketones, heteroaryl alkyl ketones, 1-alkenyl alkyl ketones, α-dialkylamino ketones, α-glycosyl alkyl ketones, cycloalkanones, 4-piperidinones, and steroidal ketones, resulting in excellent yields. Notably, when an additional acidic functional group is present, this reagent can also aminomethylenate it and in some instances, this can lead to ring closure. The reagent itself is a bench-stable compound, typically presenting as a colorless to slightly yellow substance with a distinct amine-like odor. It is miscible with nonpolar aprotic water-free solvents such as benzene, toluene, cyclohexane, and diethyl ether, but reacts with protic solvents and even common solvents like acetonitrile or acetone which are weakly CH-acidic react on heating
Synonyms: 1-(1,1-Dimethylethoxy)-N,N,N′,N′-tetramethylmethanediamine (ACI); Methanediamine, 1-tert-butoxy-N,N,N′,N′-tetramethyl- (7CI, 8CI); 1-tert-Butoxy-N,N,N′,N′-tetramethylmethanediamine; [(tert-Butoxy)(dimethylamino)methyl]dimethylamine; Bis(dimethylamino)-tert-butoxymethane; Bis(dimethylamino)methyl tert-butyl ether; t-BAE
Selected publication
1. tert-Butoxybis(Dimethylamino)Methane.
Kantlehner W.; Bowers A. Encyclopedia of Reagents for Organic Synthesis 2007. DOI: 10.1002/9780470842898.rb350
